Current Location:HomeGMP Pilot ProductionADC
Healsun Biopharm has 500㎡ of production line of ADC DS & lyophilized powder in compliance with FDA, EMA, NMPA, and cGMP requirements. It can produce ADC drugs with different conjugation methods according to customer requirements.
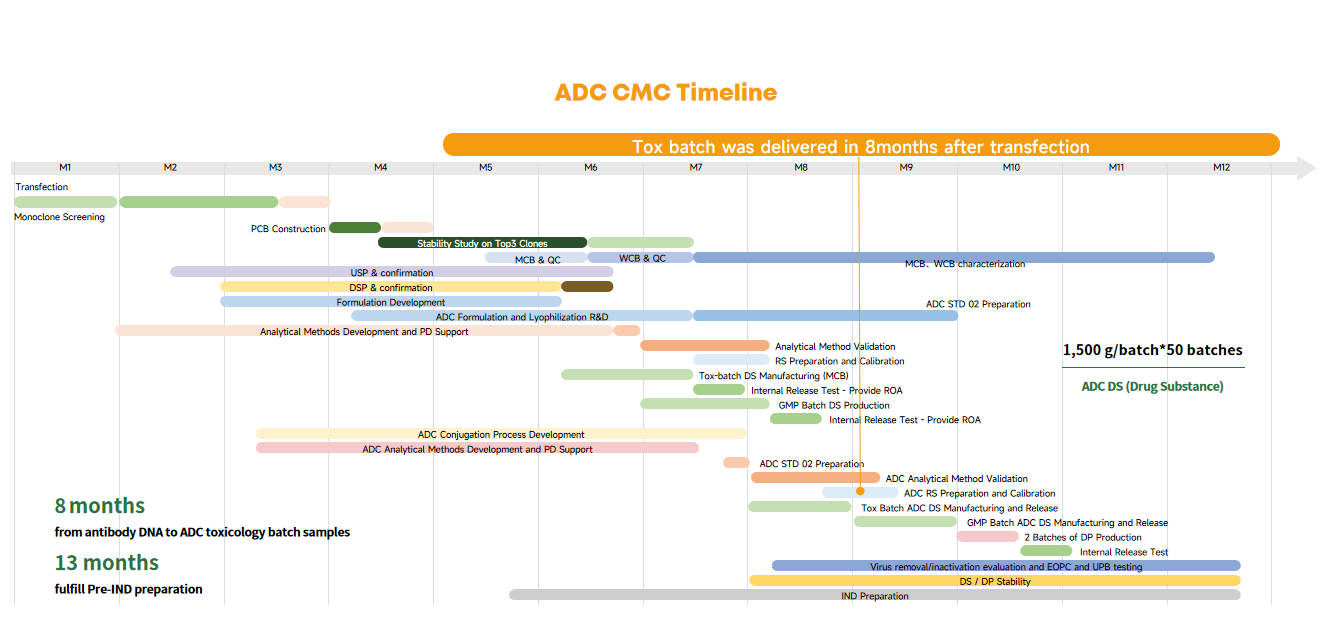
The annual production capacity can reach up to 50 batches, with each batch providing 1,500 grams of ADC.
They can deliver from antibody DNA to ADC toxicology batch samples 8 months and fulfill Pre-IND preparation within 13 months.


Email:healsunbd@hs-biopharm.com


Copyright © 2023 杭州皓阳生物技术有限公司All Rights Reserved
浙ICP备16013229号-1
 浙公网安备 33011002011493号
技术支持:杭州网站制作
浙公网安备 33011002011493号
技术支持:杭州网站制作