Healsun Biopharm started its journey by providing stable cell line development services and has gradually expanded its scope of services to one-stop R&D and production solution for antibodies, fusion proteins, and ADC products from protein engineering, early druggability evaluation, CMC process development to commercial production.
Early-Stage Development
Stable Cell Line Development
Process Development
GMP Pilot
Production
GMP Commercial Production
CGT
Our purpose is to help identify and optimize potential drug candidates in a quick way.
More>>Our Haoyue®皓越 Cell Line Development Platform, based on commercially licensable CHO-K1 host cells and proprietary high-expression vectors, provides faster and more robust cell line development services, ensuring optimal product quality and reducing development timelines and commercialization costs. HaoYue® 皓越 Fut8 Knockout Cell Line Platforms is developed from commercially licensable CHO-K1 host cells. This platform enables the generation of homogeneous and stable low-fucosylation modified cell lines through transfection and screening, resulting in enhanced ADCC (Antibody-Dependent Cell-Mediated Cytotoxicity) effects.
With our QbD-based process development capabilities, we can accelerate your drug development process.
· 5000㎡ of GMP production line of DS (Drug Substance) and DP (Drug Product ) in compliance with FDA, EMA, NMPA, and cGMP requirements Production service of toxicology batch samples, GMP samples for IND filing and clinical trials.
· 500㎡ of production line of ADC DS (Drug Substance) & lyophilized powder in compliance with FDA, EMA, NMPA, and cGMP requirements.
Biologics :6*2,000L;
ADC :1* 500L;
Sterile filling of liqiud & lyophilization production line :50,000 vials/batch;
Pre-filled syringe production line :100,000 vials/batch;
ADC sterile filling of liqiud & lyophilization production line :20,000 vials/batch.
Providing full-process AAV/mRNA CRO services, exploring all possibilities in technology.
We are a one-stop CDMO provider, specializing in delivering high-quality R&D and customized services in terms of technology, facilities, and processes. Our extensive experience and expertise make us adaptable to various project requirements. And our dedication to providing services without competitive conflicts allows us to build stronger trust and collaborative relationships with our clients.
One of the earliest companies in China to conduct systematic druggability evaluation based on CMC requirements. Systematic druggability evaluation provides comprehensive data and insights for drug development teams, guiding candidate molecule confirmation, process, quality control, and stability design. This reduces the risks in later-stage development, contributing to the success of drug development.
Our team has over 15 years of experience in stable cell line development, and innovative approaches and excellent technical development ensure high yields and stability of stable cell lines. Supported by an efficient, high-throughput automated stable cell line development platform, we can deliver stable cell lines for symmetric structure mAbs/bsAbs with a minimum production of 5g/L under conventional 14-day fed-batch process. We also have extensive experience in the development of complex molecules such as non-symmetric structure bsAbs/trisAbs, fusion proteins, etc.
Successfully delivering over 200 sample conjugation preparations annually, with rich project experience in the ADC field. Proficient in various conjugation techniques and toxin molecules, capable of selecting and applying the most suitable technical strategies based on different drug properties and targets. Thanks to our comprehensive ADC characterization platform, we are able to fully understand and assess ADC properties, and to provide in-depth analysis and interpretation to our clients.
Equipped with comprehensive GMP production lines for antibody DS and DP, as well as a dedicated GMP production line for ADC DS, we are capable of delivering antibody and ADC toxicology batch samples from DNA within 7 and 8 months, respectively. Our exceptional speed and efficiency help clients initiate the IND phase quickly, gaining a significant advantage in the critical stages of drug development.
Our experienced management team with a wealth of project expertise in drug development and production efficiently plan project workflows and maintain close collaboration with clients throughout each stage, ensuring timely communication of project progress, risks, and decisions to facilitate the smooth advancement of projects. Their rigorous quality management processes guarantee that every project complies with international standards and regulatory requirements at all stages.

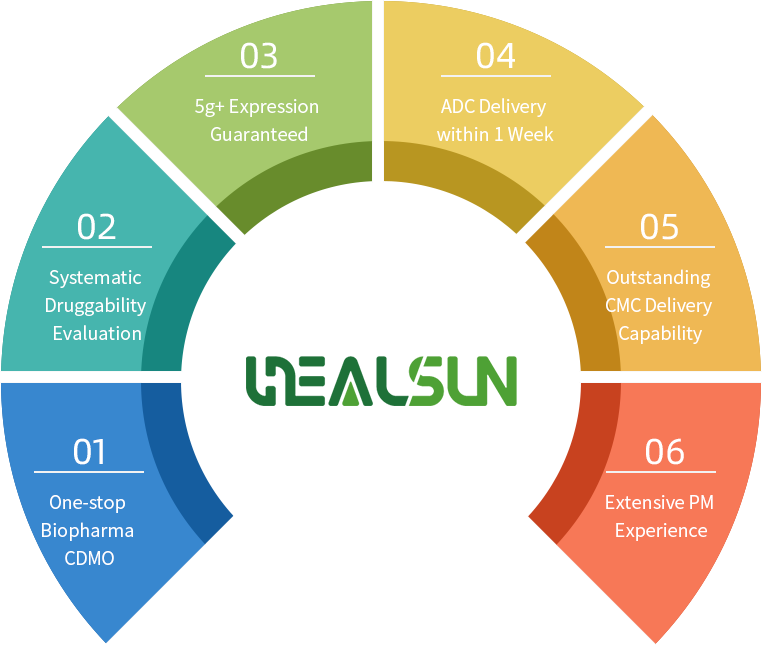
One-stop Biopharma CDMO

Systematic Druggability Evaluation

5g/L Expression Guaranteed
ADC Delivery within 1 Week

Outstanding CMC Delivery Capability
Extensive PM Experience
Founded in 2015, Healsun Biopharm started its journey by providing stable cell line development services and has gradually expanded its scope of services to one-stop R&D and production solution for antibodies, fusion proteins, and ADC products from protein engineering, early druggability evaluation, CMC process development to GMP commercial production.
160 +
Delivered CLD Projects
1300 +
Delivered ADC Projects
35
Projects in IND Phase





