Current Location:HomeBiosimilars
1. Benefits of Biosimilars
Biosimilars are medicines that are highly similar to currently available biological drugs, known as “reference medicines”. They can be marketed once the reference medicines have lost their exclusivity rights and patent protections.
Biosimilars offer a range of benefits to multiple stakeholders:
① Cost Savings:
Biosimilars offer cost-effective alternatives to expensive biologic drugs, making treatment more affordable for patients and healthcare systems.
② Increased Access:
They enhance patient access to essential therapies, especially in regions with limited resources.
③ Competition:
Biosimilars foster competition in the pharmaceutical market, encouraging innovation and potentially lowering drug prices.
④ Market Expansion:
Expanding the range of treatment options through biosimilars can help biotech companies enter new markets and reach a broader patient base.
⑤ Healthcare Sustainability:
Biosimilars contribute to the sustainability of healthcare systems by reducing the financial burden associated with biologics.
2. CMC development
Unlike the development of innovative antibody drugs, the biggest challenge in the development of biosimilars lies in the CMC development part. Because for biosimilars, it is much more complex. At the beginning of the project, it is necessary to fully study the various quality indicators of the originator, then quality comparison studies should be carried out throughout each stage. Therefore, the development timeline of biosimilars has become a touchstone for CMC development capabilities.
Similar to the CMC of general biological drugs, the key and difficult points in the development of biosimilars also lie in:
① Cell line construction:
Biosimilars have higher demands for both expression levels and quality attributes. High expression is a potent tool for cost-efficient production, and the pursuit of quality attributes necessitates precise alignment with the host cells used by the reference drug, because host cells have an important impact on the protein quality.
② Upstream and downstream process development:
During the final clone selection phase, joint participation of upstream and downstream experts is essential. Quality attribute issues related to glycosylation could be adjusted by upstream processes, while issues related to acid-base isomerization and fragments or dimer could be addressed simultaneously by both upstream and downstream processes.
③ The development and validation of analytical methods boasting requisite sensitivity and precision
3. Our biosimilars
Our biosimilars covering different therapeutic areas such as diabetes, oncology, hematology, ophthalmology and immunology, demonstrate exceptional similarity to reference medicines. With notably higher expression levels than reference medicines, our biosimilars promise cost-efficient future commercialization and improve drug accessibility. We have successful experiences in IND application (Our Dulaglutide biosimilar has made history as the 1st to receive IND approval in China).
At Healsun, we help build your own biosimilars. Our extensive experience and expertise make us adaptable to various project requirements. And we are dedicated to providing services without competitive conflicts.
If you are interested in any of these projects or other ones, please contact us for further discussion. We look forward to our collaboration.
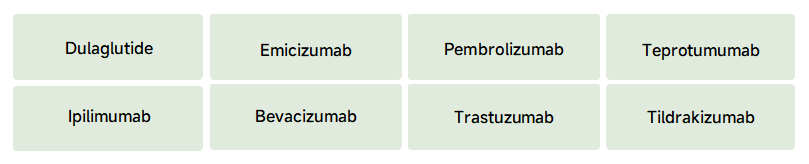
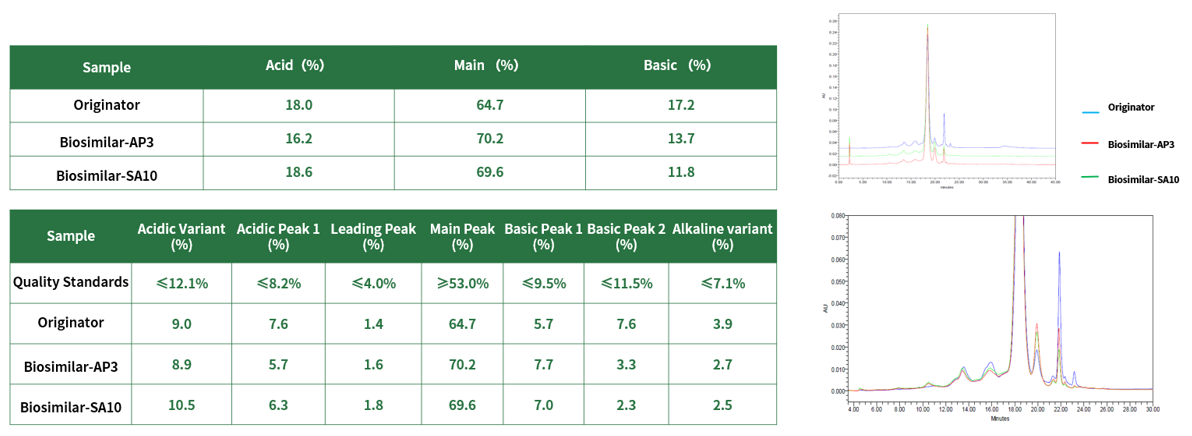
4. Some data of our biosimilars of Pembrolizumab(Keytruda)
AP3 & SA10 HSP10XX-20230804 Bioreactor Data:
CEX-HPLC:

Email:healsunbd@hs-biopharm.com


Copyright © 2023 杭州皓阳生物技术有限公司All Rights Reserved
浙ICP备16013229号-1
 浙公网安备 33011002011493号
技术支持:杭州网站制作
浙公网安备 33011002011493号
技术支持:杭州网站制作