
Bispecific antibodies (bsAbs) are non-natural antibody structures capable of simultaneously recognizing two sites or binding to two antigens. They are composed of two minimal antigen-binding fragments connected by a linker, which is further connected to the IgG structure, resulting in a "large" molecule that can cross-link additional domains. Through innovative scientific approaches, a variety of intricate bsAb structures have been created, endowing them with the potential of achieving a "1+1 greater than 2" effect in theory. bsAbs possess unique characteristics and the potential for cocktail therapy, surpassing the capabilities of parental antibodies expressed alone or in combination.
In the field of tumor immunotherapy, which is one of the most extensively explored areas, bsAbs can simultaneously bind to two sites, exerting effects such as blocking tumor cell signaling pathways, facilitating immune cell-mediated killing of target cells, and mitigating the "off-target" effects associated with monoclonal antibodies. This multifunctionality helps reduce tumor cell escape mechanisms. Owing to their diverse advantages, bsAbs have garnered significant attention from the industry in recent years and have emerged as a focal point in the development of various diseases, including cancer, inflammation, and autoimmune disorders.
With the advancement of the industry, several bispecific antibodies (bsAbs) have been approved and launched in the market, marking significant milestones in the field. This includes Catumaxomab as the pioneering product, followed by the approvals of Amgen's Blinatumomab and Roche's Emicizumab. More recently, the National Medical Products Administration (NMPA) officially announced the approval of Candonilimab, a PD-1/CTLA-4 bispecific antibody, for commercialization. These achievements indicate a promising future with the emergence of additional bsAb therapeutics.
However, successfully transitioning a bsAb from laboratory development to clinical application and ultimately benefiting patients necessitates strategic design and comprehensive considerations from the perspective of Chemistry, Manufacturing, and Controls (CMC). Such an approach encompasses various aspects, including formulation development, production scalability, quality control, and regulatory compliance, to ensure a seamless journey from the laboratory to the clinic and ultimately to the market. By adopting a robust CMC strategy, the potential of bsAbs can be fully realized, paving the way for transformative advancements in patient care.
Through careful analysis and structural characterization, bispecific antibodies (bsAbs) can be categorized into two main types: IgG-like bsAbs with an Fc region and non-IgG-like bsAbs without an Fc region. Within the IgG-like bsAb category, there are symmetrical and asymmetric structures. Representative examples of IgG-like bsAbs include Catumaxomab and Emicizumab. This type of bsAb offers advantages in terms of improved solubility and stability. However, complex structures within this category may present challenges such as increased aggregation, mismatching, and lower yields.
On the other hand, Blinatumomab belongs to the category of non-IgG-like bsAbs. This type of bsAb offers the advantage of having a simpler structure and generally higher expression levels. However, specific purification technology routes need to be developed to address the unique characteristics of non-IgG-like bsAbs.
Successfully navigating the development of bsAbs during the Investigational New Drug (IND) stage requires careful consideration of the following key aspects:
I. Cell Line Development
Considering the Quality by Design (QbD) principles and practical factors, the significance of an "exceptional" cell line in the entire CMC process is undeniable. It serves as the starting point and foundation for the entire CMC workflow. An efficient and stable cell line possesses key attributes such as high expression levels, excellent product quality (low mismatching/aggregation), and good stability. These characteristics directly impact subsequent process development, production scalability, and cost control. In the development of 15 bispecific antibodies (bsAb) projects between 2021 and 2022, Healsun has accumulated experience in seven different structural formats, as depicted in Figure 1.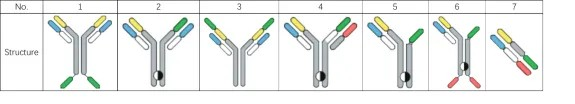
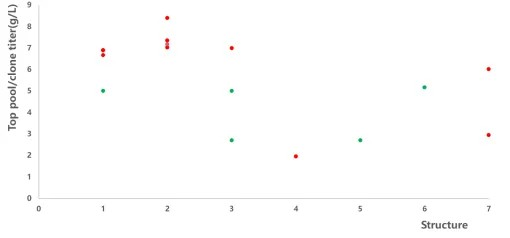
Figure 1. bsAb -expression summary
Note: The commercial CD medium is a conventional 14-day Fed-batch culture process; the red dot is the Top clone titer; the green dot is the Top pool titer.
Currently, in the context of bsAb cell line development, Healsun has taken a multidimensional approach, considering various factors and designs. This primarily encompasses the design of expression vectors, selection of host cells, and the design and selection of detection methods and screening approaches.
Customized vector construction strategies for different structural formats of bsAbs were implemented. In the early stages of cell line development, high-throughput assays were employed to assess the purity and matching rate of bsAbs. Key indicators and technical points were determined during the cell line screening process to maximize the chances of identifying cell lines expressing superior bsAbs. An example of the development of an Asymmetric format bsAb is outlined in Table 1
Following transfection and high-throughput mini pool screening, a population of cells was obtained, and the expression and quality characteristics are summarized in the table below.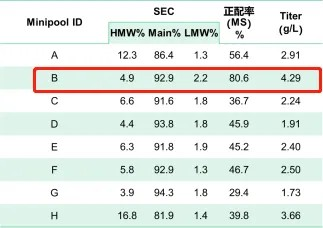
Table 1
Among them, mini pool B exhibited significant advantages in terms of matching rate and expression level compared to other mini pools. The size-exclusion chromatography (SEC) purity was within an acceptable range. Consequently, mini pool B was selected for monoclonal screening. The expression and quality profile of the cell line after one-step affinity purification are presented in the table below.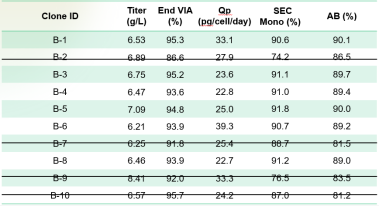
Table 2
This demonstrates that obtaining a cell population with high matching rates and superior expression levels in the preliminary stages greatly increases the probability of subsequently identifying an ideal cell line. This significantly reduces the challenges in downstream process development for the Asymmetric format bsAb.
II. Upstream Process Development
Undoubtedly, obtaining a cell line that expresses high-quality proteins provides significant advantages for the subsequent development of bsAbs. However, it is not uncommon to encounter situations where an ideal cell line cannot be identified. Cell line development is a time-consuming and labor-intensive bottleneck step. Repeating this process brings about high uncertainty, making it challenging to consider time and economic costs. Upstream process development can intervene in a timely manner to optimize product quality through cultivation condition improvements. An example of such development is presented below.
For the Symmetric format bsAb shown in Structure 1 of Figure 1, the complexity of the molecular structure can lead to protein cleavage during secretion, and the fragments cannot be effectively removed through purification. Various companies have adopted different approaches to address this issue for the popular target structure. For instance, one approach involves modifying the cytokine sequence to reduce cleavage (WO2018205985A1). In this particular project, high-throughput cell line screening did not result in significant improvement in cleavage without sequence optimization. However, after selecting the top clone, combined efforts in medium development and cell culture process optimization successfully reduced the proportion of bsAb cleavage and effectively controlled fragment content, leading to increased purification yields. The subsequent pilot-scale production achieved yields of 65%-70%, which were comparable to monoclonal antibodies.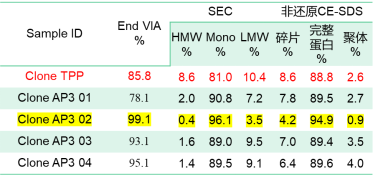
Table 3.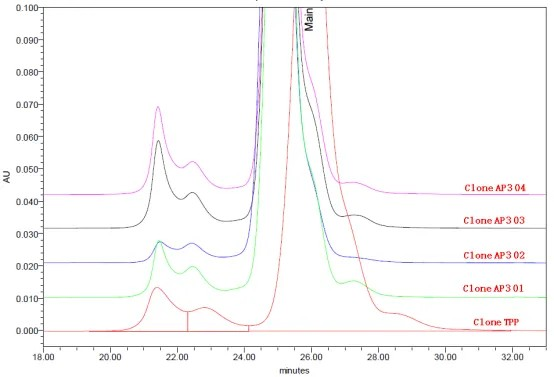
Figure 2. Symmetric formats bsAb SEC overlay:TPP is clone screening, AP3 01-AP3 04 are 3L bioreactor culture under different culture conditions
III. Downstream Process Development
Compared to monoclonal antibodies, bsAbs exhibit a more diverse molecular structure and a greater complexity of by-products, such as homodimers, heterodimeric Fc species, half antibodies, light chain dimers, and heavy chain dimers, which pose higher challenges for downstream purification processes. Currently, the purification strategies for bsAbs still heavily rely on chromatography techniques. Unlike the classical three-step chromatography strategy for monoclonal antibodies, bsAbs do not follow a fixed purification protocol. A successful bsAb purification strategy must simultaneously consider the removal of by-products, the yield of the target protein, cost considerations, and process scalability.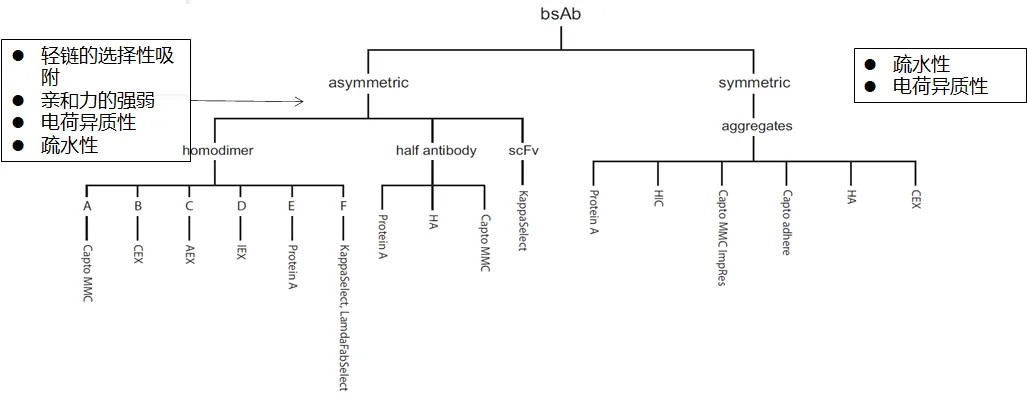
To rapidly and effectively develop a bsAb purification strategy, it is essential to understand the molecular structure and properties of the target protein. Only by knowing yourself and the enemy can you be invincible in all battles. bsAbs often possess unique molecular designs to improve correct pairing and minimize by-product generation. At times, increasing the differentiation between the bsAb and by-products can enhance the resolution of chromatographic steps. Additionally, based on established platform methods, appropriate chromatographic processes are selected to remove impurities.
Finally, the overall ability of the process to remove impurities is considered, and a purification strategy is formulated. Typical purification processes involve capture, intermediate purification, and final purification steps. These principles can also be applied to the development of bsAb purification strategies by assessing the ability of each chromatographic step to remove different impurities, considering input conditions and output results for each step, and effectively connecting various chromatographic processes to achieve the production of qualified products. An example of the development of an Asymmetric format bsAb is provided below.
Through high-throughput cell line screening, a cell line with high expression levels was obtained, but the protein quality differed from the original research. By implementing downstream processes to remove mismatched molecules and performing purification, the protein quality was highly similar to the original research.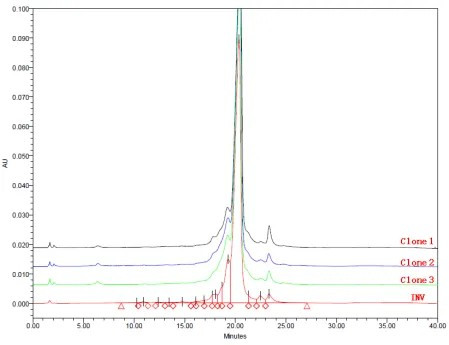
Figure 3. Asymmetric formats bsAb CEX overlay
As a burgeoning concept in the field of antibody therapy, bispecific antibodies (bsAbs) have garnered sustained and remarkable attention. Several companies have strategically positioned themselves in the field of bsAbs, and although most are currently in the research and development stage, it is foreseeable that the bsAb landscape will become increasingly crowded in the future. Overcoming the various challenges encountered in the industrialization process of bsAbs requires collaboration and comprehensive consideration from all parties involved. We believe that in the future, more efficacious bsAbs with lower side effects will enter the market, providing patients with greater choices and hope.
Reference
[1] Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18, 585–608 (2019). https://doi.org/10.1038/s41573-019-0028-1
[2] A roadmap for lgG-like bispecific antibody purification,DOI:10.1016/B978-0-08-103019-6.00008-4,(2020)
[3] Alkaline cation-exchange chromatography for the reduction of aggregate and a mis-formed disulfide variant in a bispecific antibody purification process, DOI:10.1016/j.jchromb.2014.11.002,(2015)
[4] Analytical characterization of a monoclonal antibody therapeutic reveals a three-light chain species that is efficiently removed using hydrophobic interaction chromatography, DOI:10.4161/mabs.26192,(2013)
[5] Current trends and challenges in the downstream purification of bispecific antibodies, DOI:10.1093/abt/tbab007,(2021)