
The definition of the word “stable” is to describe things that are firmly fixed; not likely to move, change or fail. The 3 elements of “safe, effective and quality-controllable” of drugs are projected onto the research and investigation of “stability”, which is given a meaningful extension to the characterization of pharmaceutical terminology. During the entire macromolecular drug development process, related research on “stability” are involved for many times. In the process of cell line construction, the starting point of CMC development, stability study also plays a crucial role in the monoclonal evaluation as a key element. It refers to the study of genetic stability of cell lines, aimed at evaluating candidate monoclonal cell lines at the preliminary development screening stage by simulating generations, processes or conditions of late-stage mass production, so as to explore and ensure the scalability of cell lines and the quality of expressed antibodies.
CHO is a classic mammalian protein expression system in the biopharmaceutical industry, and its source and history have been thoroughly reviewed and summarized in many articles, so we will not repeat it here. As a good research model with fewer chromosomes than common experimental animals (CHO was initially reported to have 22 chromosomes), the heterogeneity of CHO cells is also widely recognized in the industry. Some studies have shown that in the 1970s, some sub-clonal chromosomes of CHO cells were reduced to 21 or even 20. Except for changes in chromosome number, other studies have revealed that CHO cell chromosomes may also spontaneously exchange and rearrangement on their own. The genes encoding APRT, LDHA and GAA enzymes on chromosome 3 of CHO cells were investigated, and the opposite positions of these genes were inverted and rearranged on the chromosome, forming a new Z4 chromosome, as shown in Figure A below. Gene recombination was also found between adjacent chromosomes 3 and 4, forming new chromosomes Z3 and Z7, as shown in Figure B.
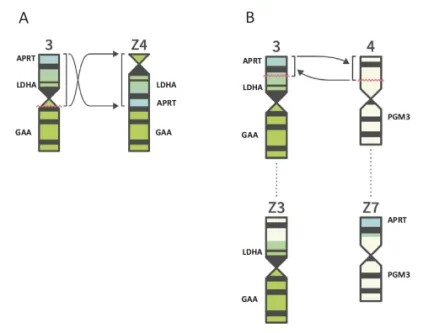
FIGURE 1 Generation of three Z-chromosomes by rearrangements.
Therefore, some famous scholars believe that due to the strong heterogeneity of CHO cells, it can be assumed that their genes are always in the process of dynamic change during continuous passaging, so there is no real “homogeneous” CHO cell bank. This characteristic of CHO cells will obviously bring uncertainty to the pharmaceutical process and antibodies to be expressed, which runs counter to the purpose of “controllable”. Therefore, from the perspective of quality control, it is particularly important to investigate the stability of cell lines. In practice, we need to examine whether the cell lines’ own growth and metabolism are traceable, and whether the expressed antibodies are homogeneous and controllable during the production window period and process cycle.
Consistent with the concept that “stable” is relative to transient expression in “stable cell lines”, cell stability studies should examine the stability of candidate cell lines under the conditions of simulating actual production processes and scales, including cellular, gene, and protein levels. We can make it clear that there are ranges and intervals for examining the stability of cell lines. And in the early cell line construction stage, the stability of the cell line is assessed to establish a Primary Cell Bank (PCB), which serves as the starting point for continuous passaging to reach the largest generation of future commercial production. The definition of “generation” may differ from company to company. In our opinion, the cell generation can be more accurately assessed by population doubling level (PDL). Since the cell division is exponential, the PDL of cells in a single passaging and culture can be calculated by the following formula:

During continuous passage, the actual PDL of the cells is the cumulative PDL of the passages, thus avoiding the uncertainty of this calculation due to the passaging frequency and time. At the start of the PCB stability assessment of cell lines, the scale and process of actual production may not be finalized. Therefore, PCB routine stability study generally simulates the actual cell bank construction and expansion process, so that the cells are passaged for 60 PDL with or without screening pressure, and whether 60 PDL is sufficient to cover the generations for 20,000 L manufacture scale is calculated. After the completion of passaging, PCB cells (defined as PDL 0) and EOPB cells (generally PDL 60) are resuscitated for Fed-batch under the same conditions. The cell line is considered stable if the cells express elevated or reduced expression within 30% and the amount of expressed protein does not drift significantly. Through diverse monoclonal screening strategies, the assessment of genetic stability of cell lines can be greatly improved.
It is worth mentioning that regarding the evaluation of cell line stability, the current regulations do not clearly define a specific PDL, or a specific rate of change in cell expression to specify the stability of a cell strain. The regulations only require that the study be carried out, and emphasize the normalization of genetic stability. For example,“Transgenerational stability study should simulate the actual production conditions (such as with or without pressure in the expansion and production stage, passage cycle, culture and expression process conditions, etc.), examine the stability at the cellular, genetic and protein levels, and limit the manufacture passage times. In particular, during NDA application, it should be assessed whether the established stability study can meet the needs of commercial scale production.” Based on this, we need to think more about the current industry practices and standards for cell line stability assessment. Due to technological advances in the industry, cell line expression has been greatly increased compared with the past. In terms of the conventional fed-batch method, is it still necessary to reach 20,000 L for actual commercial batch production scale? Under the premise of quality control, does a change of more than 30% in the expression of a cell line necessarily mean that it is unstable? All these are worthy of our attention and further discussion, but in any case, the purpose of cell line stability evaluation is very clear. With the in-depth research on host cells, the development of technology and the increase of evaluation methods, based on quality control, cell line stability research will certainly be more scientific and comprehensive, and give more accurate tips for the later commercial production stage. Just like skilled fortune-tellers who read the coffee grounds to uncover hidden insights, these advancements in cell line stability research will enable us to foresee and navigate the challenges of future pharmaceutical manufacturing, ensuring a brighter and more successful tomorrow.
Reference
1、 Maria J Wurm, Florian M Wurm. Naming CHO cells for bio-manufacturing: Genome plasticity and variant phenotypes of cell populations in bioreactors question the relevance of old names. Biotechnol J.2021Jul;16(7):e2100165.
2、 Siciliano, M. J., Stallings, R. L.,&Adair, G. M.(1985). The geneticmap of the Chinese hamster and the genetic consequences of chromosomal rearrangements in CHO cells. In: Gottesman, M. M.[ed] Molecular Cell Genetics, John Wiley and Sons. 95–135.
3、《生物技术药物研究开发和质量控制》(第三版),王军志主编,科学出版社
4、周莉婷,罗建辉。《生物制品上市申请药学申报资料常见问题和审评关注要点分析》。 《中国新药杂志》2020,29(03):264-268.