
1. Introduction
If we compare drug research and development to a vast ocean, each of our process is like a submarine sailing into an unfamiliar sea, and analysis and testing is the sonar system of the submarine, which guides us in the right direction through the results of each test, Therefore, it is crucial for us to have an excellent “graduation defense” of analytical method.
2. Concept of method validation
The purpose of analytical testing is to obtain stable, reliable and accurate results. Through the Pharmacopoeia of various countries and various types of guidelines for the definition of method validation: method validation is to prove that the analytical method can meet the requirements of the intended purpose through a scientific way. As we said at the beginning, only the analytical method which has passed the “graduation defense”, is the method we need, and can become our “eyes”.

3. Content of method validation
The content of method validation is consistent across national regulations which include specificity, precision, accuracy, linearity, range, and robustness. The contents to be validated are selected on a case-by-case basis, depending on the purpose and characteristics of the assay as well as the object of analysis.
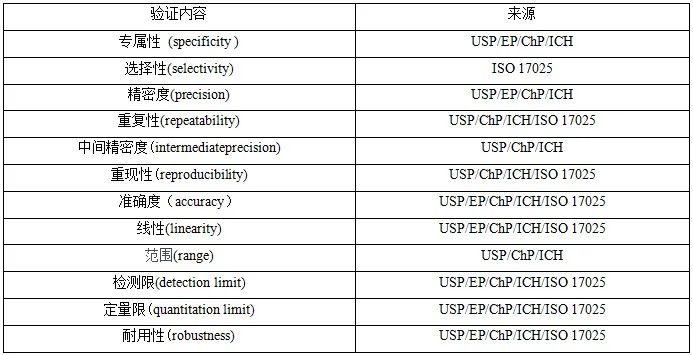
Specificity
Specificity refers to the ability to determine analytes accurately and reliably in the presence of certain components (e.g., impurities, degradation products, substrates, etc.). Its validation samples are usually selected as impurities, related substances, samples by forced degradation, and buffer solutions after preparation.Experiments are typically performed as: resolution experiments; negative control sample experiments; assays of samples with impurity reference (degradation products) added; result comparison of samples containing impurities/degradation products with those by another validated method; and peak purity determination.The focus should be on the interference of impurities and related substances in the sample, substrate effects (the substrate in which the analyte is located and that to be analyzed). And specificity is especially critical for identification and impurity detection.
Accuracy
The accuracy of an analytical method refers to the degree of similarity between the true value or recognized reference value and the measured value. The sample is usually a sample/impurity standard, and either direct measurement or spike recovery is common practice.
The experimental format is to prepare the same concentration (equivalent to 100% concentration level) of the test sample and evaluate it with at least 6 parallel determinations, or to design 3 different concentrations, high, medium and low, and prepare 3 separate solutions of the test sample for each concentration level and evaluate it with 9 determinations.
The most common form of outcome evaluation is by recovery rate, while differences and confidence intervals can also be used as evaluation tools.
Precision
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility. Repeatability expresses the precision under the same operating conditions over a short interval of time. (Since the sample preparation process is part of the method, it needs to be distinguished from instrumental repeatability.)
Intermediate precision expresses within-laboratories variations: different days, different analysts, different equipment, etc.
Reproducibility expresses the precision between laboratories. (Reproducibility should be examined for method standardization and is often used in the transfer of methods.)
Repeatability of the experimental design is defined as 6 parallel assays at the same concentration with the same experimenter, instrument, and time, or 3 different concentrations at high, medium, and low levels, with 3 assays prepared at each concentration level, and the design of repeatability should be considered in conjunction with the accuracy, linearity, and range components. Intermediate precision is the comparison of results between different experimenters, instruments, and times.
Results are usually evaluated in the form of statistical calculations such as standard deviations, coefficients of variation, and confidence intervals.
Linearity
The linearity of an analytical method refers to the proportionality of the detection result to the concentration (amount) of the analyte in the sample within a given range. The experimental design linearity routine is not less than five concentration points for experiments, and the general range is not less than 80% to 120% of the target concentration, thus obtaining linearity between assays and sample concentrations.
Results are usually evaluated in the form of correlation coefficients, slopes, intercepts, residual sum of squares, etc. Deviations of measured values from the regression line also contribute to the evaluation of linearity results.
Note: In special cases, it is possible to convert the results (absolute value/square/radical sign, etc.) and then perform a linear regression calculation. It is even possible to construct a nonlinear model, as long as it can respond to the concentration-response relationship.
Range
The range of the analytical method is the concentration interval of the sample that meets the requirements for accuracy, precision, and linearity
LOD and LOQ
Limit of detection (LOD) of an analytical method is the lowest amount of analyte in a sample that can be detected. Limit of quantitation (LOQ) is the lowest amount of analyte in a sample that can be accurately quantified, and shall meet the requirements of accuracy and precision. Conventional experimental formats are usually direct calculation method and signal-noise ratio method.
Direct calculation method: The lowest concentration or amount that can be reliably detected/quantified using a known concentration of the measured substance/test.
Signal-noise ratio method: the signal measured from a known low concentration sample is compared with the signal measured from a blank sample, and the lowest concentration or amount of the measured substance that can be reliably detected/quantified is confirmed by calculating the signal-noise ratio. Generally, the signal-noise ratio of LOD is not less than 3:1, and the signal- noise ratio of LOQ is not less than 10:1.
When special circumstances arise, the slope method can be used to calculate the LOD and LOQ by establishing a standard curve according slope and the remaining standard deviation.
Robustness
The robustness of an analytical method is defined as the ability to test samples unaffected when test parameters are intentionally altered in small ways. Method robustness should be examined throughout the method, starting with sample preparation. Conventional aspects to examine are instrument, material, method, process, environment, etc. Column temperature, bandwidth, pH variation, ambient temperature, batch variation, sample storage time, etc. are all very common factors to be tested. According to the differences in the analytical methods and the nature of the samples, the number of repeated sampling, sample processing time and other factors affecting the detection results should be considered.
We have briefly summarized the contents of method validation. Different kinds of methods should be examined in different contents, and there are clear requirements and guidelines in the regulations. Method validation is part of our understanding of the method and sample quality control, so in the method validation, we should follow the actual needs to design the method validation program in order to achieve the desired purpose of the test. Here we select the specificity and LOD for further analysis.
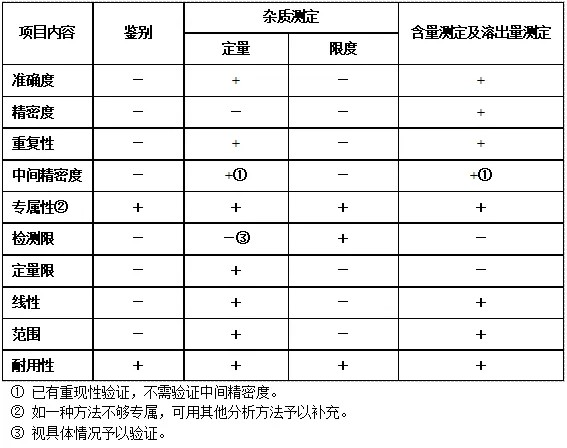
Specificity
Distinction between specificity and selectivity
Before examining specificity, the distinction between specificity and selectivity should first be clarified. In USP<1225>, it is suggested that some international institutions prefer the term “selectivity”, reserving “specificity” for the methods that are fully selective.
①Specificity means that an analytical method produces a unique signal for only one analytical component;
②Selectivity is the ability of the analytical method to accurately detect the component to be tested when other components are co-existing in the test article. (Reserving specificity for the methods with full selectivity).
However, in practice, specificity is like a utopian existence, and few analytical methods are truly specific. Therefore, we should use selectivity rather than specificity. Selectivity is also divided into detection selectivity and resolution selectivity, which we will not discuss here.
Meaning of resolution
In the investigation of specificity, the resolution experiment is a common experimental method. A correct understanding of the meaning of resolution and rational use of specificity validation is of great significance. Theoretically, when R=1.5, the two adjacent peaks which obey normal distribution are equal in height, they can be considered to have been completely separated, and “the resolution degree between the chromatographic peaks of the substance to be tested and the adjacent peaks should be not less than 1.5”, which is the standard from ChP Volume Ⅳ General Chapter 0512.
However, in the actual process of method validation, the chromatographic resolution and concentration of the components to be tested, the complexity of the test material system and different purposes of the assays lead us to fail to obtain a good linear chromatography. And this phenomenon is especially obvious in the process of biologics assay, so we should formulate a reasonable standard of resolution according to the actual situation instead of copying the standard.
It is herein additionally proposed that, when the relevant substances of certain samples are more numerous and complex, in addition to stipulating the resolution degree of adjacent peaks, the resolution degree of two non-adjacent peaks may also be stipulated. The purpose is to ensure that components with retention times in between are separated by limiting the degree of resolution of the peaks of these two components without interfering with the determination of these two components.
Limit of detection (LOD)
The definition of LOD is easy to understand, and the operation and standards are relatively simple. The parts to be discussed here are the components of the LOD and the reporting of the results.
Composition of LOD
LOD consists of three parts: Instrument Detection Limit (IDL), Method Detection Limit (MDL), and Sample Detection Limit, which are combined to make a complete examination of LOD.
IDL is the ability of an analytical instrument to detect the smallest signal (generally one that can be distinguished from noise). The IDL does not consider the effect of any sample preparation step, and therefore, its value is always lower than MDL and is generally not used for final data reporting, but mainly for statistical analysis of the data and comparison of the performance of different instruments.
MDL is not only related to the LODs of different instruments, but also depends on the influence of various aspects of the whole process of the method, such as sampling, preparation, assay conditions, analyzers, environment, the nature of the sample, different instruments, etc., i.e., the total sum of errors brought about by the whole process of the method. And MDL is a relative standard to measure the detection ability of different laboratories, analytical methods and analyzers. MDL is a relative measure of the ability of different laboratories, analytical methods, and analysts to perform the test. It is generally required to be provided in the final data report and is used to indicate the uncertainty and limitations of the data.
SDL is the smallest amount that can be detected relative to the blank sample, and can only be equal to the method limit when there is no interference from the blank sample, but in practice, SDL is much larger than MDL, which is particularly evident in capillary electrophoresis.
Report on results
When our assay is a relative quantitative method, such as the use of Size Exclusion Chromatography (SEC) in monoclonal antibody analysis to detect the content of high molecular weight variant, the final LOD result is a percentage, and then we cannot just report the final percentage. With different amounts of the sample, the absolute amount of the percentage value will be changed accordingly. When reporting the result, it should be clear up to what percent at what sampling volume. For example, LOD for this method is 5% of the HMW at a sampling volume of 30mg.
Conclusion
From the first method validation in the IND stage to the comprehensive method validation in the NDA stage, we will find that method validation is not once-and-for-all. With more knowledge of the quality attributes of the samples, the optimization and change of the production process, the validation, supplemental validation and re-validation of the analytical methods are carried out through the whole drug registration cycle. For analytical methods, method validation is both the end and the beginning. After all, just like the words of “people look at the mountain, fish peep at the lotus”, we have been in pursuit of more excellent, stable, accurate and reliable analytical methods.