
CHO cells serve as the most dominant host cell line to express therapeutic antibodies, and currently more than 70% of therapeutic antibodies are produced by CHO cells. Meanwhile, gene editing technology represented by CRISPR/Cas9 is the simplest and most effective method to modify cell genome nowadays. Therefore, gene editing technology has been generated to modify CHO cells.
01 Introduction to Gene Editing
Since Watson and Crick proposed the double helix model of DNA structure in the 1950s, molecular biology has developed rapidly, advancing our understanding of genes from macroscopic level to molecular level. Starting from the invention of Zinc finger nuclease (ZFN) gene editing technology in the 1990s, until the invention of Transcription activator-like effector nucleases (TALEN) and CRISPR/Cas9 gene editing technology at the beginning of this century, mankind has been available to the Creator’s power.
Currently, there are two types of gene editing technologies: CRISPR/Cas9 and others. Since its creation, CRISPR/Cas9 technology has become the most “popular” gene editing tool with its unparalleled simplicity and convenience. The Nobel Prize in Chemistry in 2020 and the 15,000 papers on NCBI have proved it. For the detailed introduction of this technology, search on the Internet and you can get what you want.
Typical gene editing tools are composed of two functional domains: a codable sequence-specific DNA binding module mediated by amino acids (ZFN,TALEN) or RNAs (CRISPR/Cas9), which recognizes the target sequence, and a DNA endonuclease-activated module that cuts DNA double-strands, resulting in DNA double-strand breaks (DSBs). After the appearance of DSBs, the cell’s own DNA repair mechanism is activated, and the broken double strands are subjected to Homology directed repair (HDR) or non-homologous end joining (NHEJ). NHEJ is highly error-prone, resulting in base deletion or insertion, resulting in frameshift mutation or the appearance of stop codon, which can damage the gene function and achieve the purpose of gene knockout. In contrast, by transferring a repair template with homologous arms at both ends of DNA broken double-strands, we can achieve targeted insertion of exogenous genes through HDR.
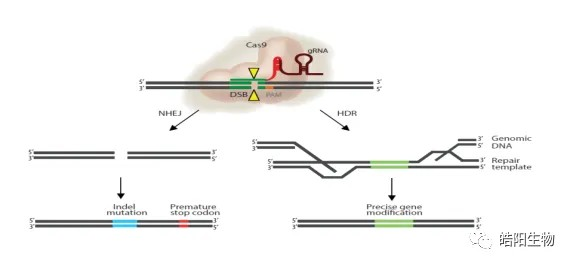
Figure 1: HDR and NHEJ fixes caused by DSBs
02 ADCC Effect of Antibodies with Fucose Modification
Antibody-dependent cell-mediated cytotoxicity (ADCC) refers to the binding of the Fab segment of an antibody to antigenic epitopes on virus-infected cells or tumor cells. The binding of its Fc segment to the Fc receptor on the surface of killer cells (NK cells, macrophages, etc.), which mediates the direct killing of the target cell. Antibodies normally bind the Fcγ receptor through the hinge region and the CH2 region. The glycan components (e.g., acetylglucosamine, mannose, fucose, and sialic acid) of the Asn-N297 glycosylation site in the CH2 structural domain determine the stability and binding properties of the CH2 structure. It has been shown that decreasing the proportion of fucose modification of Asn 297 significantly increases the affinity of the antibody for CD16 (Fcγ receptor IIIa) on killer cells and ultimately increases ADCC effects.
The fucosylation level of antibodies can be reduced by adding inhibitors of enzymes involved in the fucosylation pathway of antibodies or fucose analogs (e.g., 2FF) to the CHO cell culture medium. But for different molecules, it is often necessary to optimize the additive time and concentration to achieve the desired level of fucosylation and the stability of these additives during process scale-up has yet to be investigated. In addition, these additives are often expensive and can significantly increase production costs.
03 Knock-out of Fut8 Gene in CHO Cells by Gene Editing Technology
How can we produce antibodies with low fucosylation quickly and stably? We learned from the paper that fucosylation of antibodies is a common process of post-translational modification of glycoproteins, and that core fucosylation will happen as well. The core fucose is an α-1,6-linked fucose added to the first N-acetylglucosamine at the reducing end of the bianntennary N-linked core pentasaccharide (GlcNAc2Man3). The fucosyltransferase Fut8 is the only enzyme which can catalyzes the formation of α-1,6 fucosylation bonds. Therefore, if the gene expressing this enzyme can be knocked out of the CHO cell genome, CHO cells can produce antibodies that are theoretically free of fucose modification.
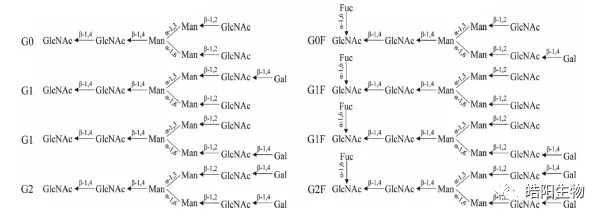
Figure 2: Schematic diagram of the common structure of antibody sugar chains
Accordingly, we used gene editing technology to knock out two fut8 alleles within the genome of CHO-K1 cells. Cells without the fut8 genes then were used for transient transfection and evaluated for titers and fucosylation levels.
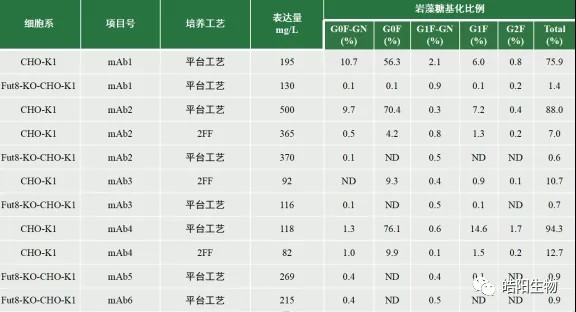
Figure 3: Antibody expression and fucosylation ratio under different cell lines and culture processes
From the above data, we can see that the wild-type CHO-K1 cell line expressed antibodies through the platform process with a fucosylation level of more than 75%, and through the 2FF process, the fucosylation level was below 15%. In contrast, cell line Fut8-KO-CHO-K1 which the fut8 genes were knocked out used a platform process to express antibodies whose level of fucosylation remained stable at less than 1.5%. From several different project molecules, we can find that the expression of both the 2FF process and the process with Fut8-KO-CHO-K1 cells is significantly lower than that of the CHO-K1 cells using the platform process, presumably due to the reduced proportion of fucose, which affects the activity of proteins related to antibody transcription, translation, or secretion within the cell, leading to a reduction in overall expression. From the mAb2, mAb3 projects, we can see that the expression level of Fut8-KO-CHO-K1 cells is not lower than that of wild-type CHO-K1 cells using the 2FF process, which can prove that the deletion of the fut8 gene does not affect the physiological activity of CHO cells in other ways.
04 Conclusion
Currently, the industry is still accustomed to constructing stable expression cell lines by random integration and improving antibody expression level and quality by culture process optimization. However, with the continuous improvement and development of gene editing technology, that operation is becoming more and more simple and efficient. The development and research of constructing stable expression cell lines by targeted insertion of exogenous genes into transcriptional hotspot regions or by regulating the amount and quality of antibody expression by knocking in or knocking out genes related to antibody transcription, translation, post-translational modification, and secretion through gene editing techniques has been in full swing. And it is foreseeable that they will be well-applied in the industrial world soon. In this paper, a good example is provided by constructing a CHO cell line that can express proteins with low fucose levels by gene editing techniques.