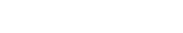Current Location:HomeCMC ProcessBiologics Process Development
Downstream Process Development Platform has wide experience in purification process development, scale-up and manufacturing for antibodies and recombinant proteins. The whole process follows the ICH Guidelines and QbD Concept, and normally achieves a highly efficient, robust and scalable downstream process in 8-12 weeks, with an overall yield of around 70%.
Equipped with intelligent protein chromatography systems such as AKTA Pure, Avant , the platform has rich experience in process development of biologics like monoclonal antibodies, bispecific antibodies, multiple antibodies, fusion proteins, ADCs.

Email:healsunbd@hs-biopharm.com


Copyright © 2023 杭州皓阳生物技术有限公司All Rights Reserved
浙ICP备16013229号-1
 浙公网安备 33011002011493号
技术支持:杭州网站制作
浙公网安备 33011002011493号
技术支持:杭州网站制作